Can stem cells be patented and how?
SUPERMAN Christopher Reeve broke his spine in a riding accident and never regained the use of his lower limbs. Until his death in 2004, he was a leading advocate of stem cell research focused on the treatment of various neurological diseases.
A turning point in stem cell therapy came in 2006 with Yamanaka and Takahashi’s discovery that multipotent adult stem cells can be reprogrammed into a pluripotent state. With these human-induced pluripotent stem cells (hiPSCs), the use of embryos can be avoided in stem cell therapy. This was also a legal break-through: hiPSCs made it possible to patent inventions involving stem cells by circumventing the use of embryos as a source of stem cells.
Since then, stem cell therapy has developed from basic research to a clinical discipline with close to 10.000 clinical studies on clinicaltrial.gov. This raises hope for new approaches to treat severe diseases, such as cardiovascular, metabolic, and neurological diseases. The rapid technological development in stem cell therapy and its high potential for therapeutic applications involves major investments in preclinical and clinical development.
To ensure a secure return on the investments, patent protection is required. Patents are also an important resource for making the important knowledge publicly known, to drive further innovation.
How to patent stem cells?
The legality of stem cell patenting has developed together with the scientific breakthroughs and has been handled differently in different jurisdictions. Here, we review the current situation in Europe and the USA.
Europe
The main deciding factor for eligibility for patent protection in Europe is the so-called “destruction of embryos”. The use of embryonic stem cells is not exempt from patentability in general, but the use of embryonic stem cells derived from destroyed embryos is not patentable. Today, there are few restrictions on patenting stem cells in Europe, as stem cells today can be generated from fetal or adult cells, without the use of embryos.
Patenting of stem cells as products (composition of matter) in Europe is done by defining a composition of stem cells with structural features which are not previously known to have a certain technical/biological effect. Those structural features can be the tissue of origin, and/or surface markers which, for example, when induced or used for enrichment, result in particularly advantageous properties (e.g. tissue repair) of the stem cell composition. Just like small molecules and other biologics, the use of stem cells for treatment of certain indications can also be patented by defining the stem cell composition and the relevant indication.
USA
In the USA, embryonic stem cells have not been excluded from patentability in general. Nevertheless, the obtained stem cells, irrespective of origin or differentiation capacity, cannot be products of nature but need to be “made by man”.
Traditionally, in the US, “anything under the sun made by man” has been patentable. This gives the possibility of patent protection for any isolated or engineered stem cells, irrespective of source or type. There has been turbulence in view of the Supreme Court of the United States Myriad decision of 2012, however, the USPTO has since harmonized their practice.
Today, eligibility (§101) rejections can be prevented relatively easily by considering whether the claimed product is the same as a naturally occurring counterpart or not. If not, the patent claims must describe those differences. If the same, this may mean that you have to claim the invention by the process of making it, or by a method of treatment. Most often however, similarly to Europe, there is a way to distinguish the invention from its natural counterpart, and thus make it patentable as a product.
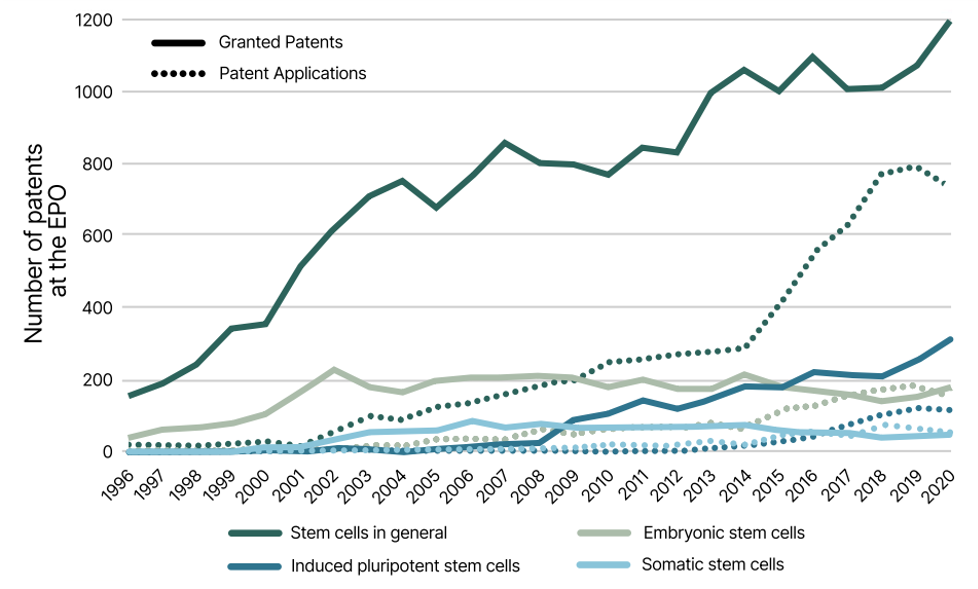
The ability to patent hiPCS has led to continuous growth and innovation in the stem cell field as can be seen in the increased number of patent applications filed (fig 1).
Stem Cell patent activity over past decades
Need to know more?
If you wish to know more about how to patent stem cells, feel free to contact our expert Jens Viktor Nørgaard.